Medicines: Is Switzerland too rich to save money?

Switzerland could save millions of francs by prescribing low-priced biotech drug copies, known as biosimilars, instead of expensive biologicals - expensive drugs produced in living cells. However, there are disincentives which are typical of the ailing Swiss system.
Emily Whitehead was suffering from leukaemia. The six-year old American girl had undergone numerous chemotherapy treatments, but nothing worked. The doctors told her parents it was time to find a place in a hospice, but then, a miracle happened. The doctors treated the girl with experimental genetically-modified human immunodeficiency viruses. Emily is now 13 and completely healthy.
This is just one of many stories about seriously ill patients undergoing treatment with new drugs called biologicals, which are produced through a biological process rather than chemical synthesis (see infobox). Such drugs, often produced involving biotechnology methods, are currently shaking up the drug industry, especially in the fields of cancer, arthritis, diabetes, heart diseases and growth disorders.
Biologicals are biotechnologically produced drugs. Biosimilars are copies of biologicals, which means they are generics. Unlike generics of chemically produced drugs, biosimilars are similar to the original product, but not identical. Hence, the approval process for biosimilars is much more cumbersome than for generics.
Even though biosimilars are still costly due to their complex production and need for approval, Swissmedic’s regulations stipulate that they have to be sold at least 25% cheaper than the original biological product.
However, such happy endings come with a cost. Developing and producing biologicals is very expensive. Even though they are rarely used for treatments in Switzerland, biologicals make up 20% of drug costs, which are soaring. This is part of the reason why health insurance premiums are rising every year and are becoming difficult to afford for many Swiss. During a recent press conference, a representative from the Helsana health insurance company warned that medical progress had its price and if we did not start to save money “we would soon be unable to afford innovation”.
Saving with biosimilars
One way to save money is the use of so-called biosimilars, which are basically generic products of biologicals. Since many patents of biologicals are expiring, Helsana sees a great saving potential in biosimilars. According to the insurer’s calculations, around CHF35 million ($35 million) could have been saved in 2016 if available biosimilars had been used instead of the originals. A forecast for 2020 even predicts potential savings of CHF300 million.
Alexander Salzmann of Sandoz Pharmaceuticals AG told swissinfo.ch: “We have done our maths, and we guess that we could save around CHF100 million or more per year.”
The catch is that, unlike other European countries, biosimilars are rarely used on the Swiss market. In Norway, for example, almost all prescribed biologicals have been replaced by biosimilars. In Switzerland, only a few biosimilars are approved, and even if they are, doctors hardly every prescribe them. But why? Depending on who you ask, there is always somebody else to blame, which is usually the case in the Swiss healthcare sector.
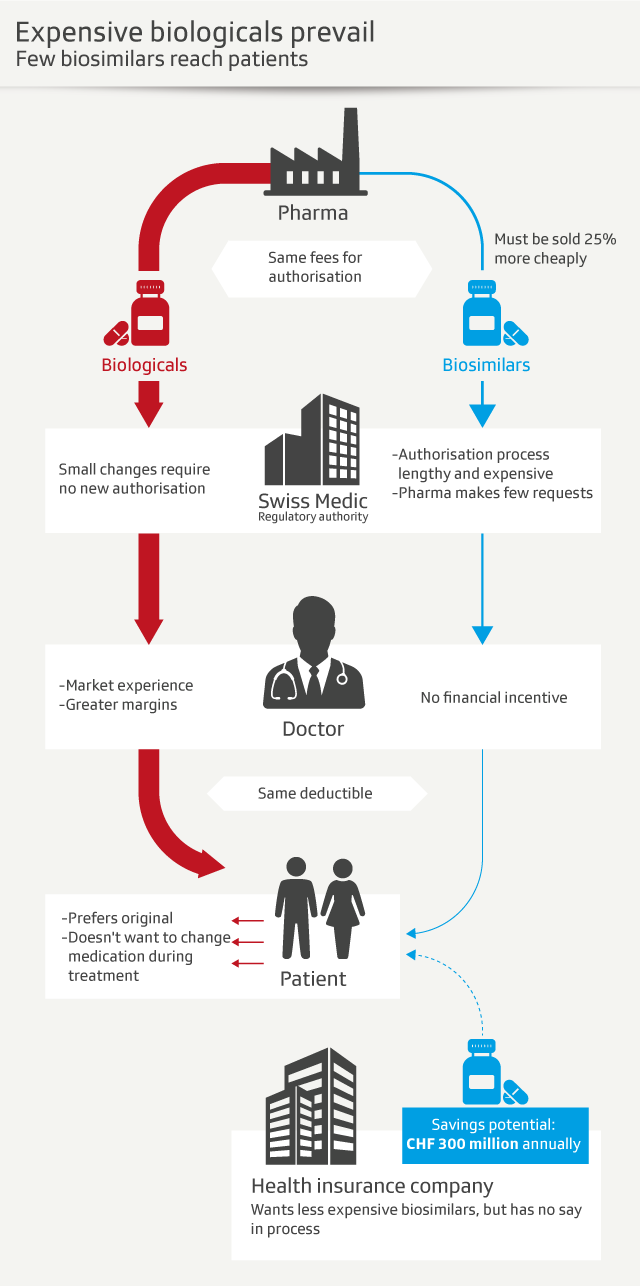
Questions and answers on why the saving potential of biosimilars is not fully exploited:
- Why do pharmaceutical companies rarely seek approval for biosimilars in Switzerland? The Swiss market is not attractive for biosimilars. The national authorisation and supervisory authority for drugs and medical products, Swissmedic, charges the same fee to approve biosimilars as for drugs containing new active substances. The approval fee for biosimilars will only be cut in 2019. At the same time, companies are obliged to sell biosimilars 25% cheaper than the original drug. What is surprising is that pharmaceutical companies can alter the production process of an original drug without renewing its approval. They must simply inform the authorities. So, it is cheaper to change an original drug than to introduce a new copy on the market.
- Why do doctors and hospitals prescribe original drugs instead of biosimilars? There is no financial incentive for doctors and hospitals to prescribe the cheaper biosimilars. It is more lucrative to keep offering the expensive originals. According to the Swiss Medical Association (FMH), doctors prefer to prescribe drugs which have been on the market longest and which they are more familiar with, as their main concern is patient safety.
- Why don’t patients ask for the cheaper biosimilars? Patients do not have to pay out a higher deductible for the expensive brand-name drug, as they do for other medicines for which generic versions exist. Pharmacists are also not allowed to substitute an expensive original biological product prescribed by a doctor or a hospital with a cheap copy, something which is possible with generic drugs. There is no legal basis for biosimilars. There are also medical concerns about changing medication during treatment.
The Federal Council (executive body) has become aware of this problem. To eliminate the disincentives, it is currently reviewing the law. “The amendments to be implemented and timeframe will depend on whether parliament passes or rejects the bill. Revising a law usually takes a minimum of three years,” the Federal Office of Public Health said.
What is the solution?
One thing is certain, this kind of legal revision is due to lead to lengthy discussions. A committee against reference prices for drugsExternal link (Gegenkomitee gegen ReferenzpreiseExternal link) has already been created. While the notion of reference prices is very popular among health insurers, pharmaceutical companies are vehemently against them.
The pharma industry is more interested in creating incentives for doctors and patients to use biosimilars. “We could offer flexible deductibles for patients, like we do for generic drugs,” Salzmann told swissinfo.ch. “I also see possibilities to introduce different insurance models.” This idea has met opposition from the health insurers.
At the press conference, Jan Triebel, deputy senior consultant of the Rheumatology Clinic at Zurich’s Triemli City Hospital, reminded that pharmaceutical companies are limited companies and are therefore keen to sell at the highest possible price for their shareholders to make a profit.
“Is it ethical to make money with human suffering?” asked Triebel, without getting an answer to his own question.

More
What’s being done to bring down high drug costs?
Translated from Germany by Billi Bierling

In compliance with the JTI standards
More: SWI swissinfo.ch certified by the Journalism Trust Initiative







You can find an overview of ongoing debates with our journalists here. Please join us!
If you want to start a conversation about a topic raised in this article or want to report factual errors, email us at english@swissinfo.ch.