Novartis leukaemia drug approved in Switzerland
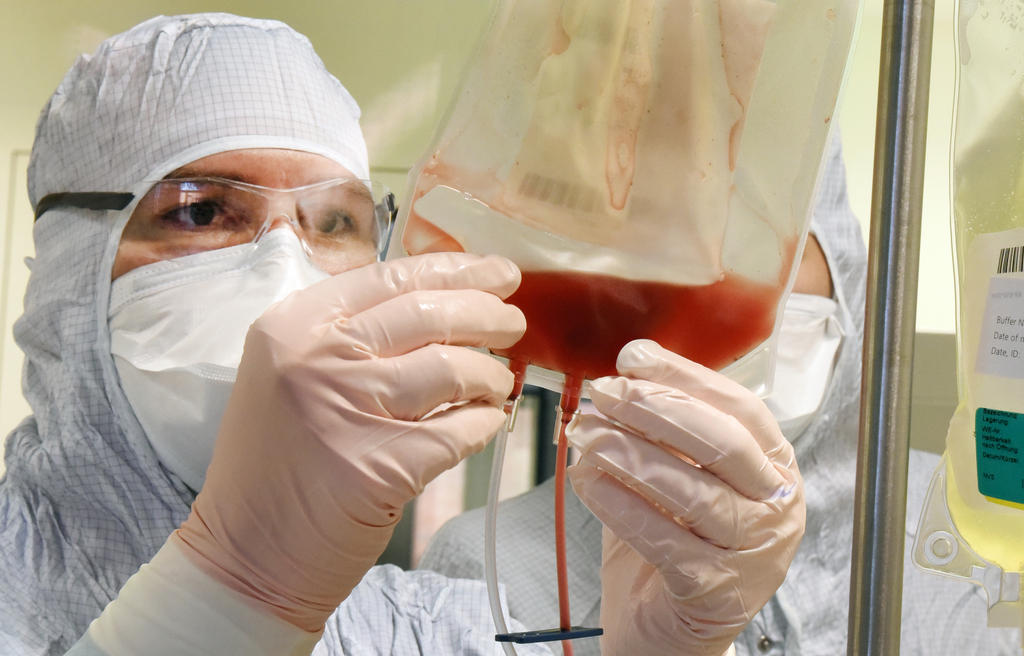
Basel-based pharmaceutical company Novartis has received approval for its personalised cell therapy Kymriah in Switzerland. Children and adults who suffer from certain types of blood cancer will be able to undergo the one-time treatment, which is set to cost CHF370,000 ($371,000).
Kymriah is already registered in the US, the EU and other countries. In the US, the treatment costs $475,000; the EU has said it will work with health officials to find fair, value-based pricing.
As in the US, pricing in Switzerland is set to be linked to the success of the therapy, although the details were still being worked out, said Kay Moeller-Heske, head of oncology at Novartis Switzerland, on Monday. The drug is not covered by obligatory health insurance, he added.
Kymriah involves white blood cells of patients being genetically modified so that they recognise and attack cancer cells. Novartis is the first Swiss pharmaceutical company to be approved for such therapy.
The number of possible treatment centres in Switzerland would initially be very limited as the Kymriah cell therapy places high demands on the production facilities and the staff, according to Moeller-Heske.
He pointed out, however, that the number of patients was expected to be very low at first: a “low two-digit number” of patients, with four or five being children.
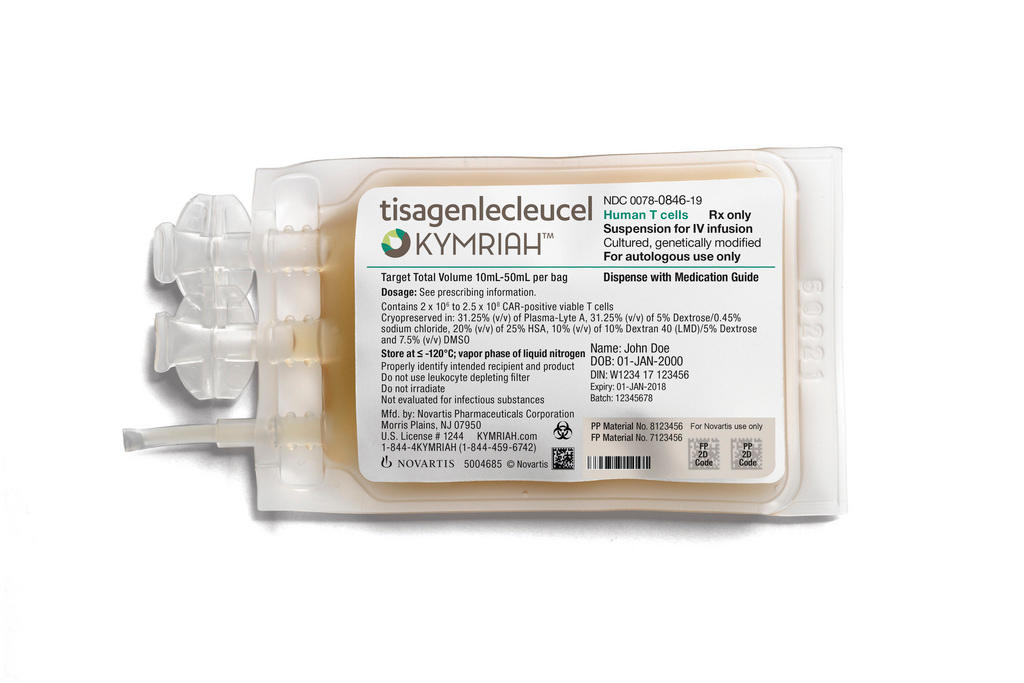
What is Kymriah and what does it do?
Kymriah, formerly known as CTL019, is a type of immunotherapyExternal link known as a chimeric antigen receptor T cell (CAR-T) therapy, and is first of its kind to receive FDA approval. Kymriah is intended for use in patients with a specific type of childhood leukaemia known as ALLExternal link. It is a one-time treatment that involves modifying a patient’s own T-cells – a type of white blood cell – and then using them to target and destroy cancer cells. Until now, ALL has had a very poor survival rate – fewer than 10% of patients survive five yearsExternal link.

More
Will exclusive collaborations yield more drug breakthroughs?

In compliance with the JTI standards
More: SWI swissinfo.ch certified by the Journalism Trust Initiative

You can find an overview of ongoing debates with our journalists here. Please join us!
If you want to start a conversation about a topic raised in this article or want to report factual errors, email us at english@swissinfo.ch.