Plugging in to blue energy
The idea of deriving power from seawater isn’t new – people have been attempting to scale up so-called “blue energy” since the 1950s. But now, a discovery from the Swiss Federal Institute of Technology in Lausanne (EPFL) could finally make energy from the ocean an efficient alternative to fossil fuel.
When you’re searching for an alternative source of energy to fossil fuels, access and abundance are two very important criteria. And in those terms, you can’t do much better than seawater, which covers about 70% of the earth’s surface.
But the secret to blue energy lies in the movement of the salt from saltwater into fresh water in a process called osmosis, which is where blue energy gets its other name: osmotic power.
“In our research, we used a concentration gradient, where we have a very high-concentration salt solution on one side of a membrane, and a solution with a very low concentration of salts on the other. This situation occurs naturally in estuaries,” explains Aleksandra Radenovic, head of the EPFL Laboratory of Nanoscale Biology.
Radenovic, together with colleagues from the EPFL Laboratory of Nanoscale Electronics and Structures and researchers at the University of Illinois at Urbana-Champaign, have developed a novel type of membrane that could give blue energy the efficiency boost it needs to join solar, wind, thermal, and hydropower in the mixture of viable renewable alternatives to fossil fuel.
Finding balance
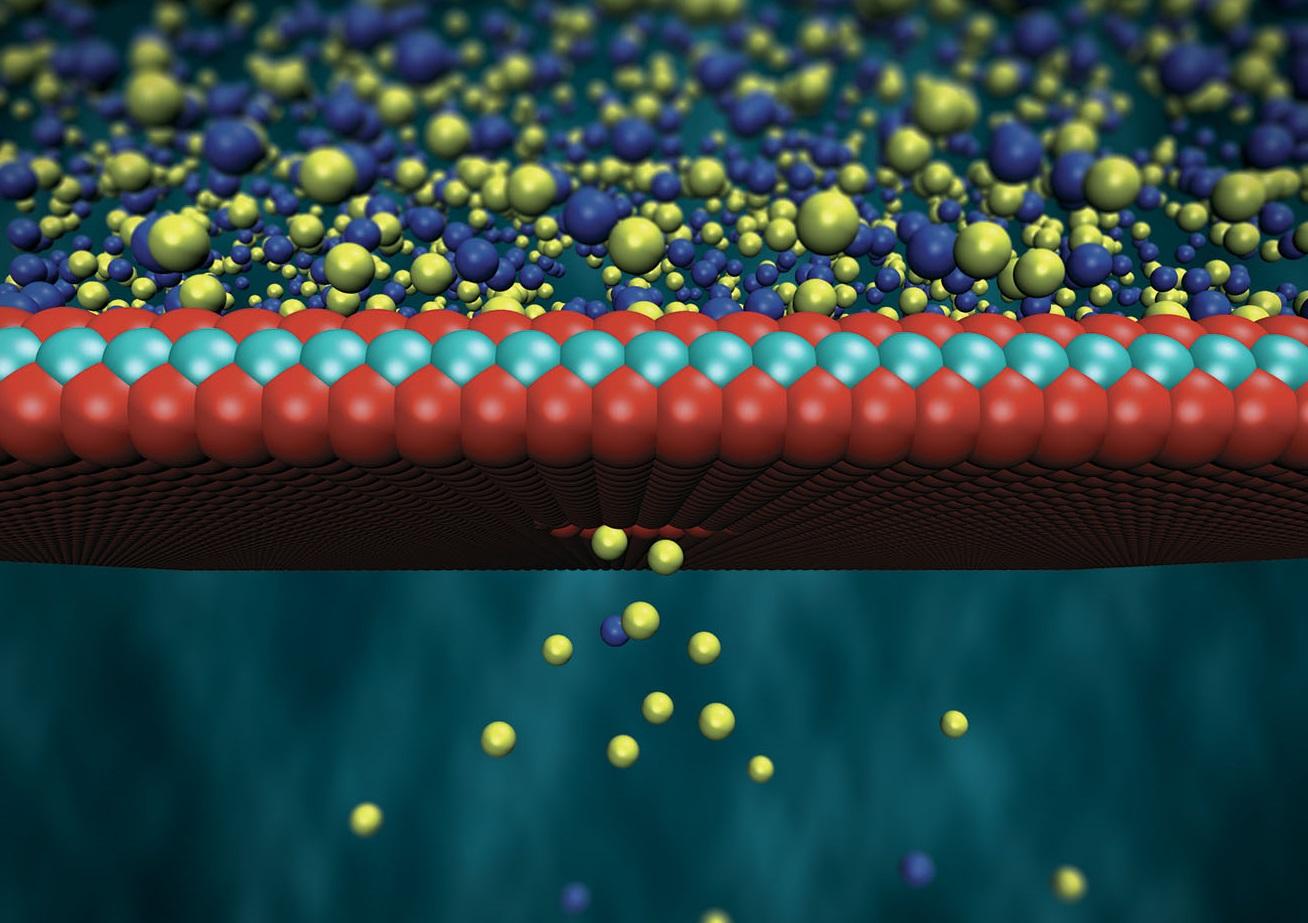
In their research, which was published in NatureExternal link, Radenovic and her colleagues describe a unique type of membrane that’s just 0.6 nanometres thick. That’s roughly equal to the thickness of three molecules, or more than 100,000 times thinner than a sheet of paper. In fact, the membrane, which is made from a readily available compound called molybdenum disulfide, is so thin that it’s classified as two-dimensional.
So what does a very thin membrane have to do with getting energy out of saltwater?
Everything, it turns out. When you separate containers of high-salt and low-salt water with a membrane, nothing happens at first. The magic lies in poking a microscopic hole, or nanopore, in that membrane, which allows electrically charged salt ions to flow naturally from the salty container into the less salty one, until the two containers reach an equilibrium.
For a more in-depth explanation of the science behind this phenomenon, check out the animation below:
The world’s first osmotic power plant, built in Norway in 2009 by Norwegian company Statkraft, used a similar type of osmosis to the one described in the EPFL research. However, it closed down in 2013 due to the company’s doubts that blue energy could be made efficient enough to compete in alternative energy markets.
Radenovic says that once it has been scaled up, their novel molybdenum disulfide system could be up to one million times more efficient than existing technologies.
Moreover, the proof-of-concept developed for the research published in Nature only has one pore in the membrane. A one-square-metre membrane with up to 30% of its surface covered in pores could produce one megawatt of electricity – 250 times the amount produced by the Statkraft plant, or enough to power 50,000 standard energy-saving lightbulbs.
Into the mix
Cost-effective scale-up and industrial application of EPFL’s blue energy technology won’t be easy though – and it’s the next challenge on the horizon for Radenovic and her colleagues.
“Our idea was fundamental research, but there are really lots of avenues where this could be explored further,” she says, adding that she’d like to see blue energy considered in the context of other renewable energy options such as solar and wind.
Advantages of blue energy over other systems include uninterrupted power – a problem that arises with solar and wind energy technologies when the weather is cloudy or the wind stops blowing.
But osmotic power plants are limited by location – they are best implemented in areas with access to the ocean, and ideally to estuaries, where seawater naturally joins up with fresh water sources. This pretty much rules out Switzerland, Radenovic says, unless of course you happen to be near a salt mine.
“I don’t envision any artificial setup for this technology, as it would not be economical,” she says. “There are many locations with abundant estuaries, especially in South America. I think that’s where such technologies should be placed.”

In compliance with the JTI standards
More: SWI swissinfo.ch certified by the Journalism Trust Initiative





You can find an overview of ongoing debates with our journalists here. Please join us!
If you want to start a conversation about a topic raised in this article or want to report factual errors, email us at english@swissinfo.ch.