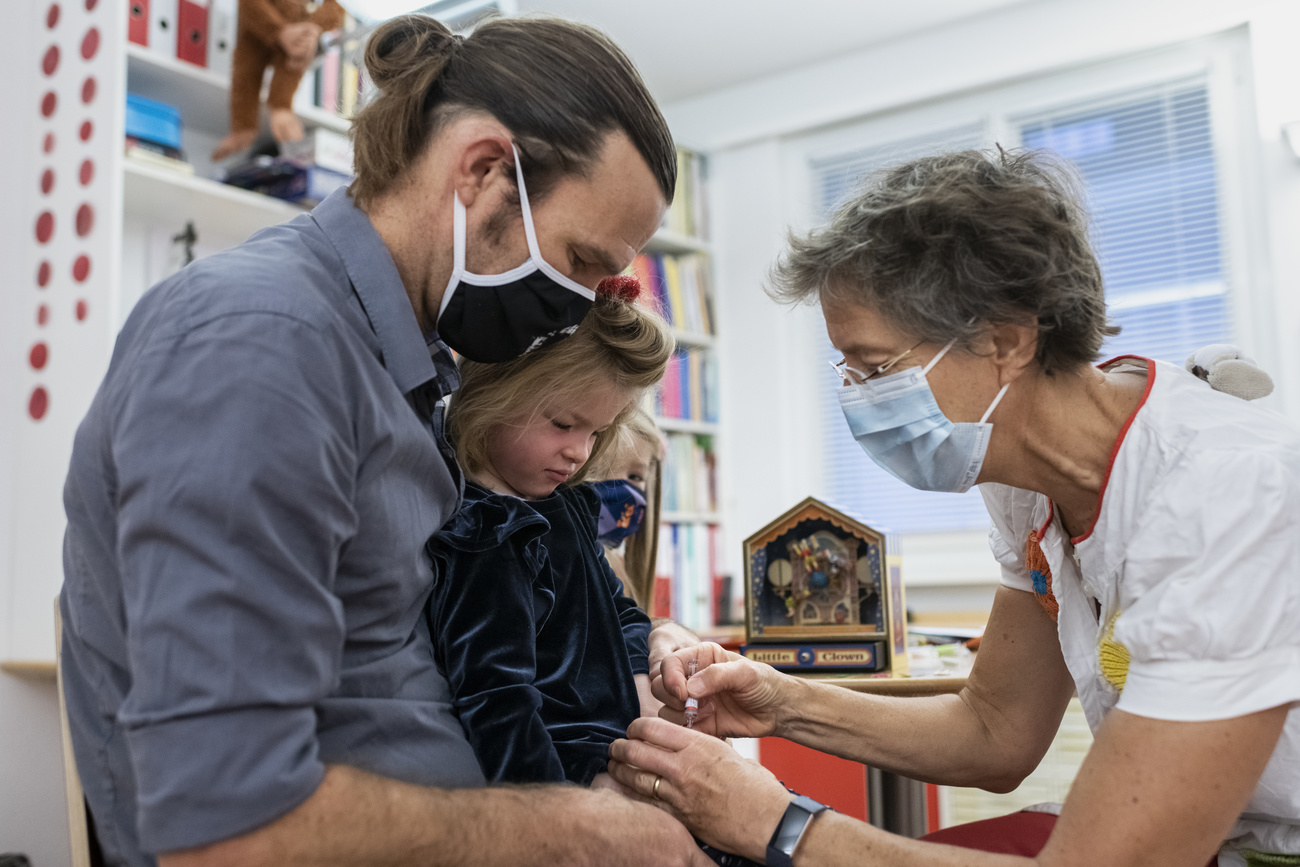
Switzerland gives green light to Moderna’s Covid vaccine for 6-11 year olds
The Swiss medical regulator Swissmedic has approved Moderna’s Spikevax mRNA Covid-19 vaccine for use by children aged 6 to 11 years.
The approval is for the vaccine’s two-dose series of 50 micrograms per dose, Moderna added. These are administered at an interval of four weeks, Swissmedic saidExternal link on Friday.
The main study in children aged 6 to 11 years found that the immune response triggered by the vaccine against the SARS-CoV-2 virus is comparable with that in young adults, the regulator said.
Around 69% of the Swiss population has received two doses of vaccine.
The Swiss health authorities recommend extending anti-Covid vaccinations to children aged between five and 11. This was introduced from January 2022. Around 6% of 5-11 year olds have been double-jabbed against Covid-19, while 8% have received a single dose, the Federal Office of Public Health (FOPH) reportedExternal link on May 9.
Switzerland is below the European medianExternal link: 39% of 5-9 year olds have received a single dose in EU/EEA countries. This ranges from 55% in Spain to 0.4% in Croatia.
The most common side effects of the Moderna mRNA Covid-19 vaccine such as pain, redness or swelling at the injection site, fatigue, headache, shivering or nausea, were similar to those in adolescents and young adults, Swissmedic said. “The undesirable side effects were generally mild to moderate and lasted for a few days,” it added.
The regulator said the vaccination was “particularly beneficial for children with previous illnesses” in whom the risk of severe Covid-19 is increased”.
According to the World Health Organization (WHO), cases of myocarditis – inflammation of the heart muscle – after receiving a Covid-19 vaccine are rare, but are reported more frequently in young persons aged 16-24 years. In February, a group of WHO experts reviewed cases of myocarditis in children and adolescents. It found that evidence of safety of the Pfizer mNRA Covid-19 vaccine was “reassuring” and that most cases were “mild and transient”. However, WHO said more information was needed about the safety of boosters in adolescents and in children if they are recommended to receive them in the future.
Moderna has received the go-ahead to administer two doses of its mRNA Covid vaccine to children aged 6-11 in over 35 countries, including Australia, Canada, the European Union and Britain.
So far, four different vaccines have been approved in Switzerland. These include mRNA vaccines from Pfizer/BioNTech and Moderna, a viral vector vaccine from Johnson & Johnson and a protein-based vaccine by Nuvaxovid.

More
Coronavirus: the situation in Switzerland

In compliance with the JTI standards
More: SWI swissinfo.ch certified by the Journalism Trust Initiative
You can find an overview of ongoing debates with our journalists here. Please join us!
If you want to start a conversation about a topic raised in this article or want to report factual errors, email us at english@swissinfo.ch.