Swiss cautious on implant removal
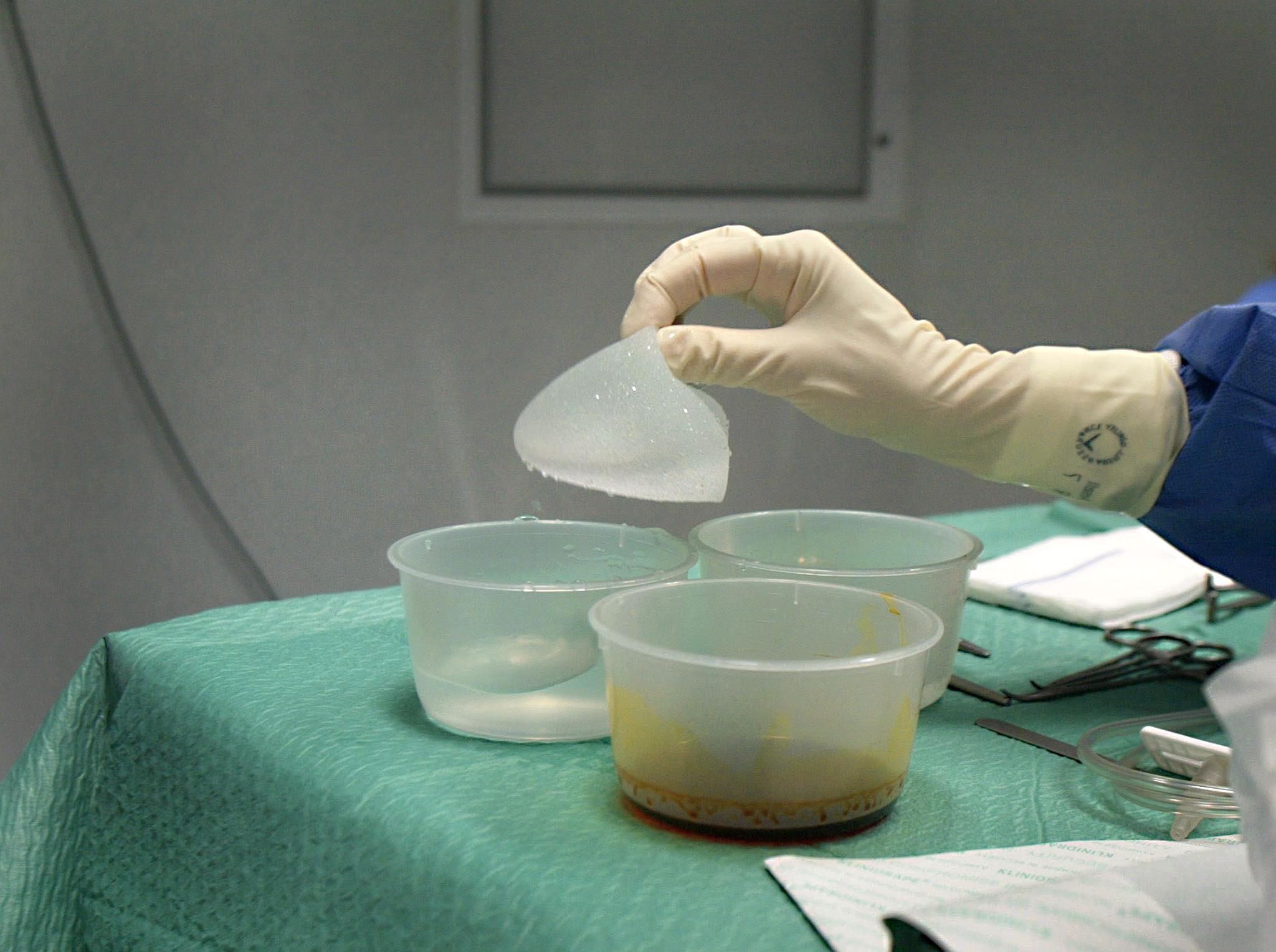
Switzerland may be adopting a cautious approach to the French breast implant scare that is worrying countries worldwide, but some Swiss women are taking action.
The Swiss medicine regulator, Swissmedic, is sticking to its line that faulty implants made by the now bankrupt French firm Poly Implant Prothese (PIP) should not be removed as a rule.
It’s a decision derided by a new Geneva association set up in recent days.
Victimes du 95c (named after a bra cup size) has criticised Swiss policy on the issue and this week filed a request for the Geneva public prosecutor to launch a criminal investigation into PIP for selling “fraudulent breast implants” in Switzerland.
Around 300,000 women around the world bought PIP implants before the company was shut down in 2010. An official inspection had revealed that PIP was using industrial, non-approved silicone in some of its products.
Tests showed the implant covers have an increased risk of rupturing – a factor which prompted France to advise 30,000 women with the implants to have them removed as a preventive measure. The Czech Republic, Germany and the Netherlands are all giving the same advice.
The number of women affected in Switzerland is miniscule in comparison, with around 280 women believed to have the implants.
But for the Victimes du 95c group, the risk is still important. According to a file sent to the Geneva public prosecutor, the association says PIP endangered women’s lives by using cheap, poor quality silicone in its implants.
Switzerland was not doing enough, the association said. “It’s like this health scare, which is preoccupying the rest of Europe, just stopped at the border.” It called on the Geneva public prosecutor to ban all use of controversial breast implants in future.
Anger
Geneva city politician Salika Wenger set up the group. “When we saw Swissmedic’s response – that this [issue] wasn’t very serious, that it hardly affected anyone – those women who had real problems were very angry.”
Wenger said she knew of a case where implants had ruptured and leaked into a woman’s body.
“There aren’t many of us, just a dozen. But since we launched, many people have called me or sent emails to say they too had problems and that they wanted to be part of the association,” she told swissinfo.ch.
“We think Swissmedic, which has the job of seeing whether products should be put on the market or not, has not done its job. We think the public authorities, especially in government, are not taking their work seriously.”
Swissmedic in fact is responsible mainly for controlling which medicines come onto the market. Quality control of medical devices, like breast implants, falls under the country’s bilateral ties with Europe, and as such follows European procedures.
In the case of the PIP implants, within Europe they were authorised by a regulatory body in Germany and given the “CE mark”, a grading that shows it meets European Commission health and safety laws.
Once a product has the CE mark it can then be distributed in Europe without the need for further checks. It’s a relaxed regime that has been criticised by doctors in the past.
“Swissmedic doesn’t check these products again. Once the notifying body says it’s ok, as a doctor you can buy this product and use it,” Swissmedic spokesman Daniel Lüthi told swissinfo.ch.
Swissmedic does intervene when products are faulty. For instance, informing patients about the problem with the PIP implants.
“The hospitals using the product have to check if there are any bad experiences, leaks or incidents, and they have to report to us if such cases happen. We exchange this information with other authorities,” said Lüthi.
Changing the status quo
Under the current system, much of the responsibility for such implants lies with the doctors, who should know “what you implant and what you explant”, Lüthi said.
The European status quo could change however in light of the breast implant scandal. Earlier this month the new head of Europe’s drugs watchdog raised the need to tighten regulations on medical devices.
“I see an urgent need to regulate devices at the same level of science and attention as with drugs,” Guido Rasi, executive director of the European Medicines Agency (EMA), told Reuters.
He said the breast implant scare could accelerate changes to rules governing the medical technology sector.
New proposals from the European Commission on regulating devices, including measures for more pre-market testing and post-marketing surveillance, are set to go before EU ministers this year.
Should that happen, Switzerland would follow suit, Swissmedic noted.
“If Europe for example decides to change anything in these recommendations then of course we have no reason not to do so because we are part of this international network,” said Lüthi.
In the meantime, the agency was continuing to discuss the issue with other countries and exchanging the latest data but there was no reason to change its guidelines just yet.
Lüthi said Swissmedic would examine the situation further should there be other “signals, medical evidence, scientific results” of ill-effects of the faulty PIP implants.
Until then its guidelines issued in December – that of women getting check-ups and seeking their doctor’s advice about implant removal – still held.
The French PIP (Poly Implant Prothese) company used cheap industrial silicone to fill the implants.
The company was banned from marketing its products in April 2010.
By that time it was producing about 100,000 implants a year, of which 84 per cent were exported, according to media reports.
They went to 65 countries, mainly in Europe and Latin America.
Company founder Jean-Claude Mas has admitted to police that at least three-quarters were filled with the cheap gel.
The French authorities found the implants had a rupture rate of five per cent; the rate is one per cent in other implants.
However, other countries have not found the same rates as in France.
When an implant ruptures, the silicone gel filling can leak into the body. Even if they do not rupture it is possible for them to leak.
This does not appear to increase the risk of cancer, but the gel can act as an irritant, causing pain.
A ruptured implant can also be more difficult to remove.
National health authorities have given different advice as to whether the implants should be removed as a precaution, since removal can also pose a risk.
France, the Czech Republic, Germany, the Netherlands and Venezuela have recommended removal, but other countries, including Switzerland and Britain, say it is probably unnecessary.

In compliance with the JTI standards
More: SWI swissinfo.ch certified by the Journalism Trust Initiative



You can find an overview of ongoing debates with our journalists here. Please join us!
If you want to start a conversation about a topic raised in this article or want to report factual errors, email us at english@swissinfo.ch.