Aids vaccine shows encouraging signs
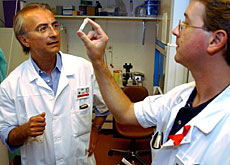
First results from trials in Lausanne and London into a vaccine to prevent Aids have been described as “promising”.
It has been well tolerated by volunteers and has prompted an immune response in almost half of them.
The vaccine, which is still in the experimental phase, was tested on 12 volunteers at the University Hospital in Lausanne and 12 at St Mary’s Hospital in London.
The first trial evaluated the safety and potency of the vaccine in terms of the generation of cellular immunity to HIV, which causes Aids.
Professor Giuseppe Pantaleo, one of the leaders of the study, said he was happy and encouraged by the results.
“We are extremely satisfied with the data we have obtained, even though this was only a preliminary study with 24 volunteers,” he commented.
Pantaleo added that he was particularly pleased that around 45 per cent of those taking part in the trials reacted to the vaccine.
The vaccine does not contain the HIV virus itself but another, non-reproducing, virus.
All the volunteers are aged between 18 and 55, HIV-negative and considered to be at low risk of infection.
Stimulating cells
In Lausanne, the vaccine stimulated cells capable of eliminating cells infected by the Aids virus in five out of the 12 people taking part in the trial.
The vaccine – dubbed NYVAC-HIV C – is the result of European cooperation and was developed under the umbrella of the EuroVac consortium.
EuroVac, the European Vaccine Effort Against HIV/Aids, has been funded by the European Union since 2000.
Twenty-one laboratories across Europe, including Switzerland, have been participating to bring European preventive HIV vaccines to Phase 1 clinical trials.
A second clinical trial, due to start in both Lausanne and London in August with 40 volunteers, is described as being “crucial” to the vaccine’s development.
“By doubling the number of people, we will have clearer and more solid statistics,” said Pantaleo.
Second phase
In the second phase, the vaccine – which has been evaluated since last year – will be combined with another vaccine that has not yet been tested on humans.
Pantaleo is hoping that 80 to 90 per cent of the volunteers will develop an immune response.
If the second round of trials is successful, tests on a much larger scale involving 300 to 400 people could be held from the middle of next year.
The vaccine was produced by Aventis Pasteur, the vaccine division of the French-German pharmaceuticals group Aventis.
In April Swiss pharmaceuticals giant Novartis lost out in its bid to take over Aventis, which accepted a $63.8 billion offer from French rival Sanofi-Synthélabo.
Lausanne has been home to the EuroVac Foundation since November 2002. The organisation aims to promote European collaboration in vaccine development, to coordinate European efforts and to raise funds for fundamental and clinical research.
With these efforts, the foundation hopes to accelerate the development of a safe and effective vaccine against HIV infection.
The foundation also promotes educational and training programmes relevant to the development of vaccines worldwide.
swissinfo with agencies
There are around 40 million people worldwide with HIV/Aids.
There were around 5 million people newly infected with HIV in 2003 – 4.2 million adults and 700,000 children under 15.
3 million people around the globe died from Aids last year.
Around 20,000 people in Switzerland are infected with HIV/Aids.
In two-thirds of cases contamination is due to heterosexual contact.
Results of a first clinical trial of a vaccine for the prevention of HIV infection have been described as “promising”.
The vaccine was well tolerated and provoked an immune response in almost 45 per cent of the volunteers who took part in testing in Lausanne and London.
A second trial, due to get underway in August, involving 40 people is said to be “crucial” to the vaccine’s development.

In compliance with the JTI standards
More: SWI swissinfo.ch certified by the Journalism Trust Initiative


You can find an overview of ongoing debates with our journalists here. Please join us!
If you want to start a conversation about a topic raised in this article or want to report factual errors, email us at english@swissinfo.ch.