Swiss start-up sets out to lower cost of gene therapy

Swiss start-up NewBiologix believes it can dramatically bring down the cost of gene therapies, typically priced in the millions, with the help of a new technology platform. It’s one of a growing number of companies setting up shop in Switzerland to meet the demands of gene and cell therapy makers.
The paint has hardly dried on the walls of the headquarters of NewBiologix at its grand opening in Épalinges, in western Switzerland on May 11. The company is used to moving quickly. In two years, it went from an idea to an 1800-square-metre, state-of-the-art facility equipped with top-notch laboratory equipment.
NewBiologix is fresh on the scene but behind the company are veteran scientists and biotech entrepreneurs. In 2001, Igor Fisch and Nicolas Mermod co-founded another company in Geneva, Selexis, that developed its own technology platforms for the production of therapeutic proteins from human cell lines (collections of cells with the same genetic material) for biological drugs. In 2017, the company was acquired by the Japanese group JSR Corporation.
Treating the untreatable
With their next venture, Fisch and Mermod are bringing their knowledge of cell line development to gene therapy, which is part of a new generation of medicine to address the underlying cause of many diseases long seen as untreatable. Gene therapy introduces into a patient’s cells genetic material intended to replace or supplement a defective gene.
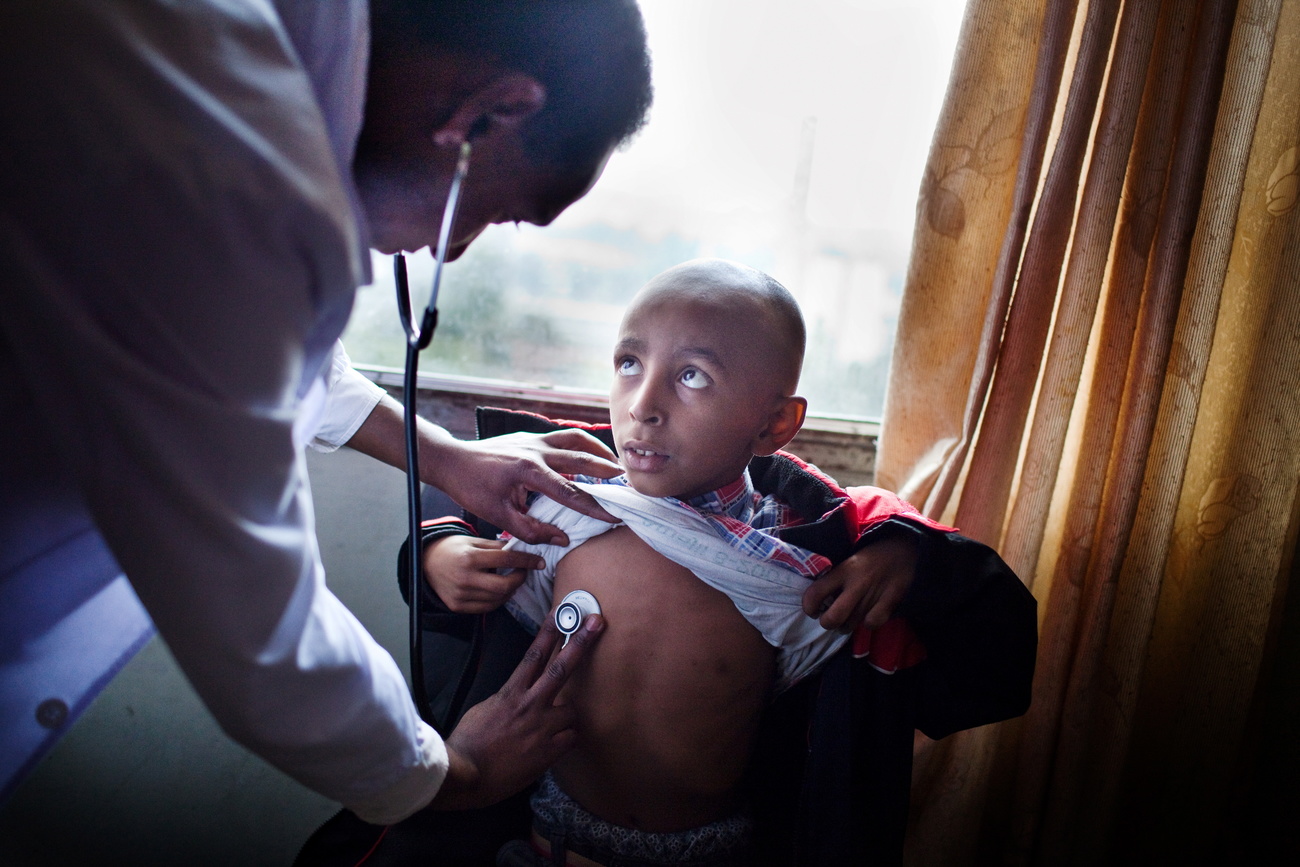
More
The end of affordable medicine
Since the first gene therapy was approved by United States regulators in 2017, there’s been an explosion of activity. There are now more than 300 gene therapy clinical trials registered by the US Food and Drug Administration to treat a range of illnesses from hereditary cancers to immune deficiency disorders.
But these therapies are massively expensive. “They are all priced in the CHF1-5 million range,” says Fisch, CEO of NewBiologix, acknowledging the challenges this poses.
Even with the high price point, some companies with successful gene therapies have struggled financially and halted production. The number of patients – fewer than 500 worldwide for some diseases – can’t make up for the high cost of development and production of the therapies. This has left patients without access to potentially life-altering treatments.
NewBiologix wants to change this. “We want gene therapy to be accessible to as many people as possible at an affordable cost,” explains Mermod, who is senior vice-president of research and development at NewBiologix and the director of the Institute of Biotechnology at University of Lausanne.
Better gene delivery vehicles
There’s very little transparency over how prices of gene therapies are determined. But a key factor in the overall cost is the complexity and cost of developing vectors. These are the “vehicles” used to transport the gene to the patient.
These are typically viral vectors, which are deactivated viruses that deliver the corrected gene into a cell during gene therapy. Producing these at scale and at a consistent quality has been a huge challenge as demand for gene therapy skyrockets.
A recent reportExternal link by McKinsey highlighted the need for viral-vector manufacturing to expand rapidly and the important role of high-yield producing cell lines.
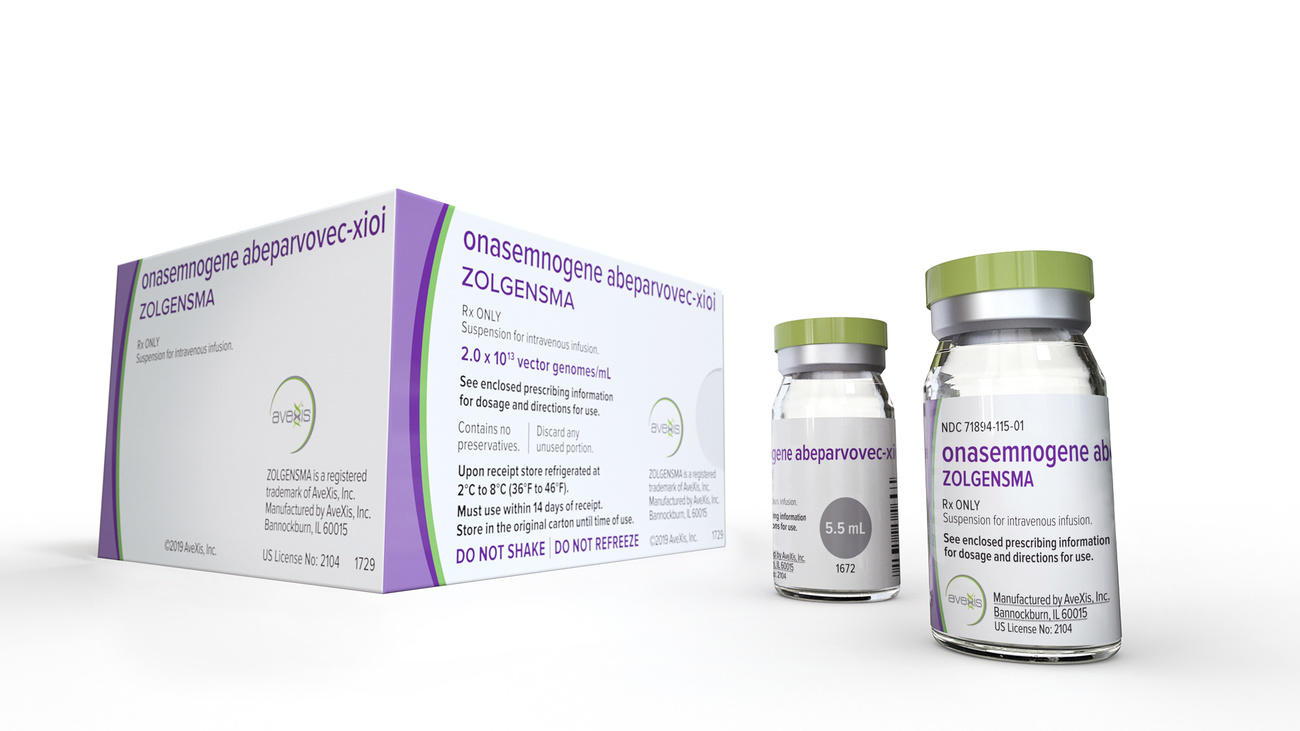
This is where NewBiologix comes in. It aims to create a more stable method to scale the production of the most common type of virus vector used in gene therapies, known as recombinant adeno-associated virus vectors. At least three FDA-approved gene therapies use such virus vectors including Novartis’s Zolgensma for spinal muscular atrophy, which carried a price tag of $2.1 million when it was launched in 2019.
The goal isn’t to produce gene therapies themselves but rather to engineer cell lines and use next generation sequencing and other tools to analyse their genome. This will allow the company to select the best cell clones among several cell candidates in order to produce viral vectors more efficiently and at lower cost.
Other companies have struggled to do this thus far because it requires specialised scientific knowledge, technical expertise, and sophisticated laboratory equipment, explains Déborah Ley, the company’s chief operating officer.
The Swedish contract manufacturer Recipharm has given NewBiologix CHF45 million ($50 million) to develop their idea. Fisch expects that by the end of 2024 the company will be ready to commercialise a product that could be licensed to companies, including big pharmaceutical firms, for developing gene therapies. By 2025, it hopes to start large-scale production.
“If we can reduce the cost of production of viral vectors with our technologies, there will be an impact on the price. Whether that will be 50, 70 or 80 percent is difficult to say,” acknowledges Fisch.
Swiss hub
NewBiologix is one of a growing number of companies in Switzerland filling gaps in the supply chain of gene and cell therapies.
A 2021 McKinsey report on European biotech firms highlighted Switzerland as one of the growth centresExternal link for new companies in the cell and gene therapy area. This includes big established players such as Novartis, which recently unveiled a new $91 million cell and gene therapy facility just outside of Basel, as well as contract manufacturers Lonza and Celonic. About a dozen smaller biotech firms including Limula and Tigen have cropped up in the last few years to help scale the production of gene-based treatments.

More
New European pharmaceutical rules met with scepticism in Switzerland
Innovation isn’t just in the specific therapies but also “in how such products are being discovered, developed and manufactured,” said Michael Altorfer, who heads the Swiss Biotech Association.
The industry is also getting some help from the country’s medicines regulator Swissmedic, which recently set up an innovation office to deal with queries about advanced therapy medicinal products such as gene therapies. This is intended to help speed up the authorisation of such products by engaging with researchers early in the process.
Edited by Veronica DeVore.

In compliance with the JTI standards
More: SWI swissinfo.ch certified by the Journalism Trust Initiative



You can find an overview of ongoing debates with our journalists here. Please join us!
If you want to start a conversation about a topic raised in this article or want to report factual errors, email us at english@swissinfo.ch.