Making medicines safe for children
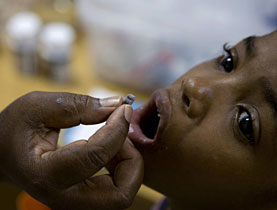
Over six million children under five die annually from diseases that could be cured if they had access to safe medicines, according to the UN children's organisation.
Experts from the World Health Organization (WHO), non-governmental organisations, regulatory bodies and the pharmaceutical industry gathered in Bern on Sunday and Monday to discuss how to “make medicines child size”.
Children in the developing world are at far greater risk of disease than those in richer countries, but the WHO estimates that even in the industrialised world more than 50 per cent of children are prescribed medicines designed for adults.
“One should realise that many medicines that are used for children have never been properly tested and have never been properly approved,” Hans Hogerzeil of the WHO told swissinfo.
But children metabolise differently from adults, and giving a child a fraction of the adult dosage may well turn out to be inappropriate and even dangerous. Furthermore, there are differences in children of various ages and body weights. Child-specific medicines have to take these differences into account too.
Medicines have a reputation for being unpalatable. Adults may be used to the idea of having “a bitter pill to swallow”, but both the bitterness and the pill present problems for children. Flavouring the medicine may be a solution to the first – but the flavour must have no impact on the curative properties. As for the second, liquids or syrups are much easier for children to swallow than pills, but the cost may be two or three times higher.
Pharmaceutical companies do little work in developing children’s medicines, not least because of the ethical problems. All clinical testing should be done with the informed consent of the patient, which is obviously difficult or even impossible with children.
In the developing world the problem is compounded by poverty. If patients are too poor to buy them or the market too small, pharmaceutical companies have no interest in producing medicines for them.
Optimism
But the outlook is not entirely bleak. The aim of the conference was to discuss ways of overcoming the problems and many participants expressed optimism.
One response is “public-private product development partnerships” which bring together companies, scientists and public money to produce the medicines which would otherwise not be made.
Hogerzeil points out that there are now a number of funds offering public money to buy medicines to fight specific killer diseases. The result is that there is more of a market.
“It’s interesting to see that in the past five or six years there is more money for malaria medicines … There is definitely more interest from the companies now because there is more money. It’s public money, it’s not the patients themselves,” he explained.
Hogerzeil sees differential pricing as an important way forward. This means that people in richer countries pay more for their medicines than those in poorer ones. In some cases there is also a difference in the prices paid for medicines supplied privately and those supplied publicly.
Clear differences in packaging make it difficult to swap them around, so that medicines supplied cheaply to one country end up being sold at a discount on markets where only the expensive version should be available.
In this way the pharmaceutical companies can cover their development costs, which poorer people can benefit from but not have to pay for.
Information sharing
Despite the fact that pharmaceutical companies are in business to make money, they also have a sense of corporate social responsibility, Yves Juillet from the International Federation of Pharmaceutical Manufacturers and Associations (IFMPA) told swissinfo.
The IFPMA announced on Monday that it had created a special Pediatric Task Force aimed at improving the industry’s contribution to making medicines and vaccines more readily available to children.
“In countries where trials are difficult to perform, it is important that when the results are available, they are shared,” Juillet explained. These results are posted on the IFPMA website and are open to all.
Many of the speakers stressed the importance of getting the information to the people who need it.
Nilima Kshirnagar, a professor from Mumbai, India, and member of the WHO advisory committee on the safety of medicines, pointed out the importance not only of communication, but also of vigilance to ensure standards are maintained.
“Access without safety monitoring would be irresponsible,” she said.
The problems may seem huge, but the “Better Medicines for Children” resolution passed by the WHO annual meeting in 2007 means they are now being addressed.
swissinfo, Julia slater
The conference “Better Medicines for Children – the Way Forward” was held in Bern on September 14-15.
It brought together representatives of UN organisations, government authorities, the pharmaceutical industry and NGOs.
It was held ahead of a conference of regulatory experts on medicinal products in Bern from September 16-19.
Many medicines given to children have not been specifically designed for them and have not been officially approved.
This applies in both the developed and developing world
The World Heath Organization is targeting five conditions for immediate action:
Acute lower respiratory infections
HIV/Aids
Malaria
Diarrhoeal diseases
Tuberculosis
WHO created a sub-committee to develop an essential medicines list for children.
It was published in December 2007 and contains 206 medicines

In compliance with the JTI standards
More: SWI swissinfo.ch certified by the Journalism Trust Initiative











You can find an overview of ongoing debates with our journalists here . Please join us!
If you want to start a conversation about a topic raised in this article or want to report factual errors, email us at english@swissinfo.ch.