
Swiss vaccine approval: As fast as possible, as slowly as necessary
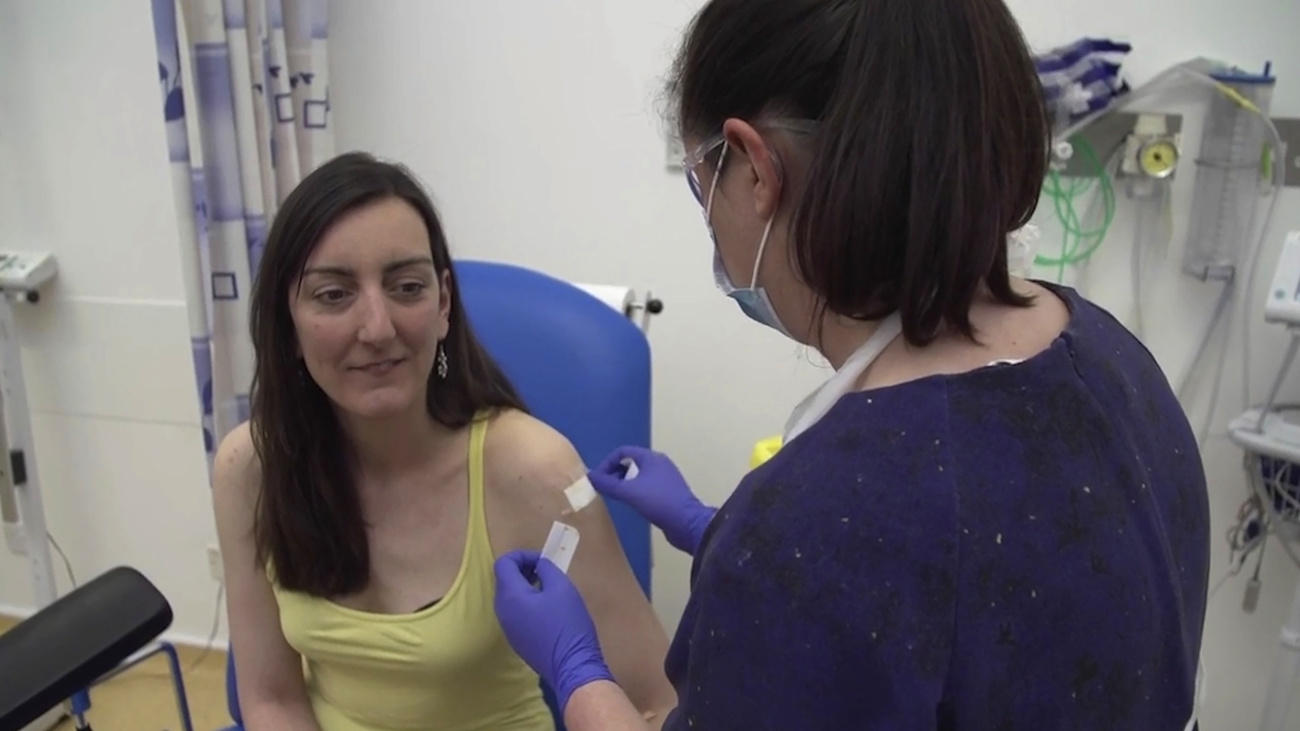
Dozens of labs around the world are working to find a vaccine against Covid-19. But they have embarked on a marathon rather than a sprint, because the approval procedures are lengthy, with good reason, including in Switzerland.
“A vaccine by autumn? No, it’s a misunderstanding. What we are hoping for is the start of phase II trials this autumn,” says Lukas Jaggi, spokesman for Swissmedic, the Swiss licensing and control authority for therapeutic products. It usually takes 10-12 years from the first laboratory tests to the arrival of a product on the market. With Covid-19, the global emergency makes it possible to speed up certain stages, but not all of them.
The scale of the pandemic has greatly boosted research into this new virus. Its genome was sequenced in record time, and on January 11 the Chinese authorities made the data available to researchers around the world. Laboratories have revised priorities to free up teams to work on the new coronavirus. And they can take advantage of technologies developed in 2003 during the SARS epidemic, a coronavirus related to the one that has brought the world to a standstill.
First tests
The World Health Organization (WHO) website lists over 100 candidate vaccinesExternal link; most are still at the so-called pre-clinical stage, i.e. testing in cell cultures or animals. The eight most advanced projects – four Chinese, three American and one British – have entered the clinical stage, that of human trials. This takes place in three phases according to strict, internationally harmonised procedures.
· Phase I: The product is tested on a few dozen volunteers who are in good health. The main aim is to check that it does not pose any major safety problems, and to detect the main side effects.
· Phase II: Testing is done this time on a few hundred patients, some of whom may be suffering from the virus. The main aim is to assess its effectiveness and determine the correct dosage.
· Phase III: Testing at this stage can be on thousands of volunteers in different countries. The objectives are to confirm the results of Phase II – that the vaccine is safe, that it works, and the dosage is correct.

More
Covid-19 is both threat and opportunity for Swiss biotech
At the end of these three phases, the product can receive a marketing authorisation (MA). But having the right vaccine is not enough; it also needs to be produced and distributed in very large quantities. So if a small start-up or a university laboratory wins the race, it will have to team up with big pharma to benefit from its firepower.
It should be noted that after getting marketing authorisation, the drug or vaccine remains under surveillance. An MA can be withdrawn, for example, if it turns out over time that the product has more undesirable side effects or that they are stronger than initially found.
Accelerated procedures
MAs are issued by each country. Whether the vaccine comes from China, the USA or elsewhere, Swissmedic will always make its own assessment, even if the product has already been authorised abroad. The Swiss authority will take into consideration trials carried out in countries with equivalent drug control.
In Switzerland, as elsewhere, people are aware of the urgency. The Federal Office of Public Health has set up a “Covid-19 vaccine” working group with experts from the government, Swissmedic, science and the economy. “The aim is to accelerate research and development and to ensure the fastest possible access to a safe and effective vaccine,” says Lukas Jaggi. “This group also wants to help ensure equitable access to such a vaccine.”
The time limits for marketing authorisation procedures are laid down in the Therapeutic Products ActExternal link and its implementing ordinance. Article 9a provides for temporary authorisation for “medicinal products for life-threatening or debilitating diseases” with a simplified procedure. And the Ordinance on Measures to Combat the Coronavirus,External link adopted as an emergency measure on March 13 to combat the virus, even provides in Article 4 that on the basis of a risk-benefit analysis, a therapeutic product may be put on the market “without authorisation pending Swissmedic’s decision”.
2021 at the earliest
But all this still doesn’t tell us when we can get vaccinated. Lukas Jaggi won’t say either, because the arrival of the vaccine depends first on who will produce and then manufacture it. The Swissmedic spokesman will say only that “the legal and regulatory instruments are in place to allow a rapid assessment, with due regard to patient safety”. Answering the question more directly, however, he adds that “if all goes well, a first vaccine could be available at best by the end of the year, and larger scale vaccinations could start in 2021, if the challenge of mass production can be solved”.
Bern lab in the race
One of the vaccine candidates, still at the pre-clinical stage, could come out of a laboratory in Bern. And it is not just any lab because JanssenExternal link, a subsidiary of American giant Johnson & Johnson, has already produced a vaccine against Ebola. The technology is the same: The common cold virus, which is unable to replicate, is combined with fragments of SARS-CoV-2 in an attempt to induce the creation of specific antibodies.

In compliance with the JTI standards
More: SWI swissinfo.ch certified by the Journalism Trust Initiative
























You can find an overview of ongoing debates with our journalists here . Please join us!
If you want to start a conversation about a topic raised in this article or want to report factual errors, email us at english@swissinfo.ch.